You will be required to submit a POM-VPS form with this product at the checkout to enable completion of your order.
Note: Currently not available for delivery to Northern Ireland, Channel Islands, Ireland Republic, EU 1, EU 2, EU 3, EU 4, EU 5, EU 6, EU 7, EU 8, Global 1 and USA.
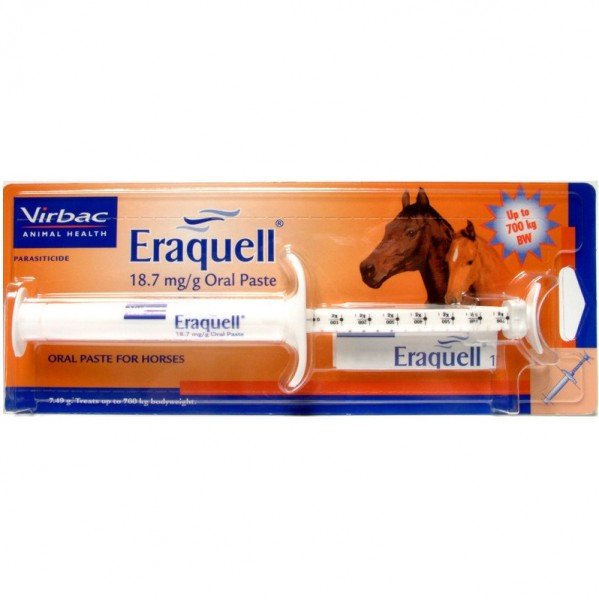
Eraquel Oral Paste Horse Wormer for the treatment and control of adult and immature gastrointestinal roundworms, lungworms and bots of horses
To ensure that you read the most up to date product information link to http://www.vmd.defra.gov.uk/ProductInformationDatabase/Search.aspx
Link to Veterinary Medicines Suspected Adverse Reaction Scheme.
Use of a scale or weigh tape is recommended to ensure accurate dosing.
Presentation
A white homogenous paste containing 1.87% w/w ivermectin Ph.Eur.
Uses
For the treatment and control of adult and immature gastrointestinal roundworms, lungworms and bots of horses, including:
Large strongylesStrongylus vulgaris adults and 4th larval (arterial) stages; Strongylus edentatus adults and 4th larval (tissue) stages; Strongylus equinus adults
Small strongyles, adults Cyathostomum spp.; Cylicocyclus spp.; Cylicodontophorus spp.; Cylicostephanus spp.; Gyalocephalus spp.
Hairworms Trichostrongylus axei adults
Pinworms Oxyuris equi adults and immatures
Ascarids Parascaris equorum adults
Intestinal threadworms Strongyloides westeri adults
Large-mouth stomach worms Habronema muscae adults
Neck threadworms Onchocerca spp. (microfilariae)
Lungworms Dictyocaulus arnfieldi adult and immature
Stomach bots Gasterophilus spp. oral and gastric larval stages
Contra-indications, warnings, etc
For animal treatment only.
Not to be used in mares producing milk for human consumption.
Some horses carrying heavy infection of Onchocerca microfilariae have experienced reactions with swelling and itching after treatment. It is assumed to be the result of death of large numbers of microfilariae.
These signs resolve within a few days but symptomatic treatment may be advisable.
Eraquell has been formulated specifically for use in horses. Dogs and cats may be adversely affected by the concentration of ivermectin in this product, if they are allowed to ingest spilled paste or have access to used syringes.
As ivermectin is extremely dangerous to fish and aquatic life, treated animals should not have direct access to surface water and ditches during treatment.
Wash hands after use.
Avoid eye contact.
Do not smoke or eat while handling the product.
Withdrawal period
Meat and offal : 30 days.
Do not use in mares producing milk for human consumption.
Container disposal
Any unused product or waste material should be disposed of in accordance with local requirements. Ivermectin is EXTREMELY DANGEROUS TO FISH AND AQUATIC LIFE. Do not contaminate surface water or ditches with the product
Pharmaceutical precautions
Keep out of the reach of children. Store below 30° C.
Legal category: POM-VPS
Marketing Authorisation Number
Vm 05653/4202
GTIN description:Eraquell 7.49g Syringe
GTIN:3597133010893
Collect in Store
This item is available for collection.